|
2 The 4 Technology Solutions

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
ENHANCED GROUNDWATER REMEDIATION, PART 1:
AVAILABLE REACTION PROCESSES
An On-Line Version of a Column First Published in:
Environmental Technology May/June 1998 Vol. 8 No. 3 Pages 24-25
By: David B. Vance
This column is the first of a two part appraisal of the current status of technology available for enhanced groundwater remediation. What will be addressed are technologies, beyond passive intrinsic remediation or the pump and treat approach. In this column we will review reaction processes that are available for use and in the next column methods of overcoming natural rates of physical mass transport available in aquifers in their natural state.
As a precursor to this discussion it is important to understand the setting of a contaminated aquifer. Almost without exception aquifer flow systems are anisotropic and heterogenetic [1][2]. This means that groundwater flow, contaminant flow, and the impact of remedial effort within the aquifer will occur through preferential aquifer pathways. Transport into zones of the aquifer bypassed by the primary flow system will be via slower advective flow, or slower yet diffusional transport. The figure illustrates this process. Complicating this process are contaminant adsorption on to the surface of particles, and diffusional transport into the clay and carbonaceous components of the aquifer matrix.
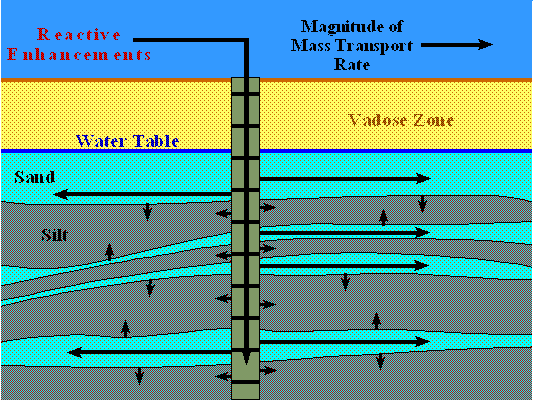
Enhanced remediation involves the injection or infiltration of biologically or chemically reactive materials into contaminated zones of an aquifer. Pump and treat is ideally not a component of the enhanced remediation process unless required by the hydrodynamics of the site or regulatory issues.
Bioremediation is the most dominant enhancement process used to date. Permutations include:
Aerobic biodegradation stimulated though the injection of oxygen by sparging with air or oxygen or using hydrogen peroxide. Aerobic degradation is effective and rapid, particularly with petroleum hydrocarbons. The problem is that oxygen is poorly transported in an aquifer because it is reactive to other components within the aquifer matrix such as iron and oxygen solubility is relatively low (8 to 10 mg/L).
Nitrate reduction is a process that is an important part of the intrinsic remediation process. However, it can be used dynamically by the introduction of nitrate into groundwater at levels less than the 5 mg/L regulatory limit. Its advantages are high solubility, mobility and low reactivity to the aquifer matrix. The first disadvantage is the selective and unpredictable degradation of specific BTEX compounds that has been demonstrated to vary from location to location. Second, is the need for pH control since every mole of nitrate consumed during hydrocarbon degradation generates one mole of sodium or potassium hydroxide that, unless neutralized with acid addition, can plug the formation with precipitated salts.
Sulfate addition could theoretically be used in a fashion similar to nitrate, it is also soluble, mobile and un-reactive to the aquifer matrix. However, the hydrogen sulfide generated as the end product of the degradation process may be unacceptable.
Methanogenesis is a process that occurs unaided in the core of a hydrocarbon plume. It is a slow process for the degradation of petroleum hydrocarbons. However, it does not rely on the mass transport of any other foreign compounds, the contaminant itself provides the reaction products and the requisite carbon load to deplete other potential competing electron acceptors within the impacted zone. In contrast, this may be the optimum biological system for the reductive dehalogenation of chlorinated hydrocarbons. In that case the addition of a supplemental carbon source can serve to drive the impacted portion of the aquifer to a methanogenic state.
Surfactant Addition is a means of addressing portions of contamination present as small blebs of free phase immiscible liquids trapped in pore spaces near contaminant release points. A surfactant will not affect that portion of a contaminant that has diffused into clay or carbonaceous particles within the aquifer matrix. In addition, surfactant effectiveness towards contaminants adsorbed to the surface of particles in the aquifer matrix is limited because the action of physical scrubbing is a critical component to remove adsorbed contaminants, scrubbing is unavailable in-situ.
Enzymatic Systems. All biological mediated activity is ultimately the result of enzymatic action. Generating those reactive enzymes in surface reactors, collecting them extracelluarly, and then injecting them into the groundwater to rapidly degrade contaminants appears attractive. Unfortunately these materials are extremely reactive, unstable and this use will require the development of soluble stabilizers (a difficult process). Some current developmental efforts are focused on cross linking reactive enzymes to stable substrates for use in above ground treatment systems particularly for the treatment of soluble oxyanions such as arsenic and selenium. The current practical in-situ use of manufactured enzymes involves a group of enzymes classed as biosurfactants, which due to their use by bacteria for extracellular reactions are stable in the groundwater environment. However, they have the same limitations of other surfactants mentioned above and are often generated in-situ as a matter of natural course by indigenous bacteria exposed to hydrocarbons.
Reductive Dehalogenation. Over the past few years the reductive dehalogenation of chlorinated solvents using elemental iron in passive reaction walls has been a technology that has garnered a great deal of attention. Now other methods of in-situ reductive dehalogenation are also being explored:
Stimulation of methanogenic conditions with the addition of innocuous carbon substrates as described above is a viable process. The combination of low redox conditions and bacterial activity are responsible for the effect.
The direct injection of hydrogen gas is being explored. Hydrogen is soluble, relatively un-reactive (when not exposed to oxygen), leaves no harmful residues, and the consumption ratio (i.e. 1 gram of hydrogen to dehalogenate 21 grams of PCE) is outstanding.
A mixture of Vitamin B12 and soluble titanium salts (such as titanium citrate) can also stimulate dehalogenation of chlorinated solvents.
There are likely to be more promising developments in this area within the next few years.
With regards to the enhanced degradation of petroleum hydrocarbons, with some site specific exceptions, it is unlikely that any currently available enhancements will have an effect much greater than natural attenuation, pump and treat, or more aggressive approaches (such as site wide air sparging.) In the case of chlorinated solvents, variations of enhancements that exploit the reductive dehalogenation process offer a significant new remediation tool.
Return to the Environmental Technology Index Page
Copyright 2008 David B. Vance
All Rights Reserved

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
|