2 The 4 Technology Solutions
![]()
If you have comments or suggestions, e-mail me at dbv7@mindspring.com
Treatment of Chlorinated Hydrocarbon Contaminated Groundwater with Injectible Nano scale Reactive Particles
By: David B. Vance
1.0 Introduction
Cost-effective, reliable technologies are needed to treat chlorinated hydrocarbon contaminants such as PCE, TCE, DCE, and CT in groundwater, especially in cases where:
- NAPL, micro emulsions or high concentration adsorbed materials are present leading to high dissolved phase concentrations.
- Access to groundwater is restricted by surface structures or uses.
- Local restrictions forbid the implementation of other available technologies such as air sparging or natural attenuation.
- Contamination is extensive and concentrations are too high for risk based closure but otherwise relatively low (typically 100-500 ppb).
- The migration of dissolved Chlorinated Aliphatic Compounds (CAHs) across property boundaries or into adjacent surface water present a long term remediation requirement.
- The vertical migration of free phase CAHs (DNAPL) into underlying drinking water aquifers is a concern.
Injectible nano scale reactive iron is a promising new technology being developed by ARCADIS Geraghty & Miller that can be used to address concentrated source areas or to install containment walls under difficult conditions, such as great depth or the presence of surface structures that prohibit trenching. Although NAPL is often envisioned as a liquid free phase, we find it is more common to have source zones (at concentrations up to 1%) containing CAH product in very fine emulsions (micro emulsions) or sorbed to the geologic matrix. Few viable technologies are currently available to directly address this type of contamination; it is often too deep for excavation and geological conditions are wrong for dewatering based techniques, such as Vacuum-Enhanced Recovery (VER). Surfactant, heat (1), (2), biological and oxidation based approaches have been tried, yet have not been widely employed for this type of source. Air sparging requires costly off-gas treatment and equipment maintenance.
Alternately, contamination can be treated in the dissolved phase; typically through ground water extraction, treatment and disposal (pump and treat). This approach requires a lengthy, costly cleanup, especially when residual CAH product is present as discussed above. In the late 1980s, air sparging was introduced as an aggressive remedy for groundwater cleanup of VOCs and has been used successfully at numerous sites. However, its application is limited in heterogeneous soils, where air sparging can disperse the contaminant plume if improperly applied. Air sparging is also a long term solution and in addition, can generate air emissions that must be controlled. Since numerous researchers have demonstrated that CAHs are reductively dechlorinated, air sparging and VER also risk the disruption of naturally occurring processes by making the system aerobic. Enhanced in situ anaerobic bioremediation of CAHs through addition of reagents is a viable approach. However, this approach will require the long term injection of reagent solution, which could prove to be slow and expensive, particularly in or adjacent to high-concentration source zones.
2.0 CAHs and Reactive Metal Processes
The use of elemental metals for in situ reductive dehalogenation has been developed over the past 8 years. Although several metals (such as zinc or tin) have been proven effective in this application, metallic iron is the preferred option due to its dehalogenation efficacy, cost, and benign environmental impact. The dehalogenation process can be best described as anaerobic corrosion. Either of the metal by the chlorinated hydrocarbon, which is adsorbed directly to the metal surface where the dehalogenation reactions occur; or by water which disassociates to produce hydrogen gas. Recent research on elemental iron systems suggests that four mechanisms are at work during the reductive process:
- First, the Fe0 acts as a reductant by supplying electrons directly from the metal surface to an adsorbed halogenated compound.
- Second, hydrogen gas is generated by the anaerobic corrosion of the metallic iron by water.
- Third, metallic iron may act as a catalyst for the reaction of hydrogen with the halogenated hydrocarbon using the hydrogen produced on the surface of the iron metal as the result of anaerobic corrosion with water. Theoretically, these reactions are not kinetically effective without a catalyst; thus, it is thought that impurities in the iron or surface defects act as that catalyst.
- Fourth, solubilized ferrous iron can also act as a reductant, albeit at a rate at least an order of magnitude slower.
The focus of the approach outlined in this white paper is an advanced, injectible application of reactive barrier/zone technology for aquifers and source material contaminated with chlorinated aliphatic hydrocarbons. Elemental nano scale bimetallic colloids that include palladium or other metals in addition to the iron can also be used for in situ reductive dehalogenation. A colloidal particle of this type is illustrated in the figure.
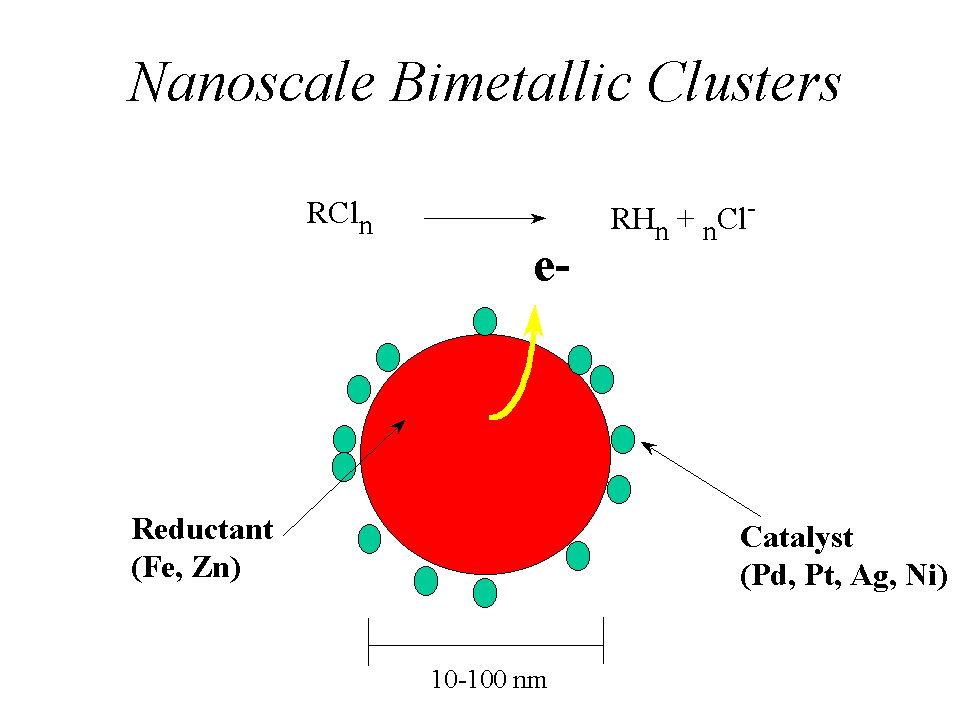
Laboratory studies have documented that a small amount of palladium coated onto the surface of a metallic iron colloid acts as an efficient hydrogenation catalyst. Our approach includes significant improvements over previous applications of the elemental iron reductive dehalogenation technology:
- markedly improved reactivity due to greater surface area of nano scale colloids (see Table 1);
- further enhancement of reactivity due to the presence of the palladium hydrogenation catalyst; and
- the ability to emplace barriers through in situ injection rather then excavation.
Table 1. Examples of the surface area of different metallic iron products
Iron type Surface area in m2/g
iron turnings 0.019 m2/g
iron 0.057 m2/g
iron granules 0.287 m2/g
commercial iron powder 0.900 m2/g
our synthesized nano scale particles 33.500 m2/g
The beneficial effects of increased surface area per unit weight and increased reaction rates due to palladium are multiplicative, giving a predicted increase of treatment rate of 100-150 times greater than that of conventional granular iron filing barriers. Due to the reduced reagent requirement and the aqueous transport properties of the nano scale colloids, in situ injection is made possible. Injection allows for direct delivery to source zones or the in situ installation of reactive barriers through injection wells at depths that are not practical using trenching methods.
Reactive barriers have been shown to offer a more cost effective alternative to pump and treat systems. Because no provision must be made for the disposal of recovered water, and the system is mechanically passive, the operational costs are 70 - 90% less. The EPA has recently published a reactive wall technology review in which they list 10 full scale field sites at which elemental iron dehalogenation was used, including many instances of successful full scale treatment of CAHs. Iron powders have been injected successfully and proven effective in treating soils contaminated with chlorinated hydrocarbons during field scale pilot tests in New Jersey.
2.1 Design Considerations for Test Site and Future Sites Including Issues of Concern
There are four design issues of concern for the proposed application of the technology:
- reagent dose and the long term life of the colloids during dehalogenation reactions and reactions with site specific constituents of the groundwater;
- the ability to generate vertical fractures in fine grained sediments;
- the ability to inject and stabilize the colloids in an effective fashion in permeable sediments; and
- manufacturing of the colloids.
2.1.1 Colloid Loading and Long-term Life
The rate at which groundwater that can be treated by a given amount of metallic colloid is directly proportional to surface area. Successful iron treatment systems have required surface area to liquid volume ratios as high as 3.5 m2/mL. Since the width of reaction zone required for any given site setting is inversely proportional to the surface area, our use of a nano scale colloid (Table 1) with a surface area 1 to 2 orders of magnitude higher than conventional iron materials offers a significant advantage. Long-term effects on colloid reactivity can be predicted through groundwater analysis, followed by avoidance of groundwater that may offer high corrosive or blinding activity, particularly in regard to the iron component of the colloid.
The issue of iron loading for the implementation of in situ colloidal reactive iron walls is driven by two primary components:
- dehalogenation reaction rates; and
- the stoichiometry of iron dehalogenation reactions.
2.1.1.1 The Effect of Surface Area and Palladium on Reaction Kinetics
Putting aside the stoichiometric issue for the moment, the reactivity of iron colloids or particles is best considered as a function of iron surface area per unit volume of water (m2 of Feo surface per ml of water).
Using a standardized surface area to volume ratio of 1m2/mL, the half lives (in minutes) for various chlorinated solvents are as follows:
- PCE 20
- TCE 110
- 1,1-DCE 650
- t-1,2-DCE 350
- c-1,2-DCE 1000
- Vinyl Chloride 830
Now, as examples, let us use the decay time of TCE which has a rigorous MCL (5 ppb), a relatively long half life (110 minutes), and an iron surface area loading of 1 m2/mL:
- At 1 mg/L TCE the time required to achieve the MCL is 880 minutes (approx. 14 hours)
- At 10 mg/L TCE the time required to achieve the MCL is 1210 minutes (approx. 20 hours)
- At 100 mg/L TCE the time required to achieve the MCL is 1650 minutes (27.5 hours)
These times can be changed by increasing iron surface loading per unit volume or by improving the kinetics. The nano scale colloids we have used have a surface area of 30 m2/g. In addition, the use of palladium on the iron colloid provides a further three times increase in degradation kinetics.
If we assume:
- a groundwater velocity of 1 foot per day;
- using an iron colloid with a surface area of 1 m2/g; and
- a CAH concentration that would require loading with iron to provide an iron surface area per unit volume of 1 m2/mL.
This would result in an iron loading in 1 cubic foot of aquifer (assuming 30% porosity) of 8.6 kilograms of iron per cubic foot (in 8.6 liters of groundwater). Increasing the surface area of the iron to 30 m2/g reduces this requirement to 287 grams. The effect of the palladium will further decrease the iron loading requirement to 96 grams per cubic foot.
To treat a portion of an aquifer 100 feet wide and 25 feet deep, with a 1 foot thick reactive would require:
- 21,500 kilograms of iron with a surface area of 1 m2/g
- 718 kilograms of iron with a surface area of 30 m2/g (239 kilograms with palladium)
A factor to consider when reviewing the analysis above is the method of application of the iron colloid. Injection through a hydrofracture approach would closely mimic this model. An exception to this would be in homogeneous tills or clays where the width of a created reactive iron zone would be limited to the width of the fracture induced by the hydrofracturing process (typically 1 to 2.5 cm). However, in such a case, either the groundwater velocity would be orders of magnitude lower, or flow would occur along the fracture (for several feet, at least) through the proppant containing the reactive iron colloid. Regardless, these changes in velocity or preferential flow direction would still allow for adequate treatment.
For intergranular injection a series of injection points on 20 to 30 foot centers would be used (or a horizontal boring). The injection process would create a reactive wall that would also be 20 to 30 feet thick (depending upon the overlap of the injection masses). The overall iron mass loading in the entire zone would be equal to that calculated for a one foot interval above. Two effects counter balance each other for treatment using the same mass, but in a larger volume:
- The amount of iron surface area per unit volume decreases 20 to 30 fold, but
- The time that it takes the groundwater to traverse the larger reactor volume increases 20 to 30 fold.
2.1.1.2 Stoichiometry
The other issue effecting iron loading is stoichiometry of the reactions with the CAHs and oxygen dissolved in the groundwater.
2.1.1.2.1 CAH Reactions
There are two primary reactions with CAHs that take place which will consume the iron and require stoichiometric consideration:
- the anaerobic iron corrosion reaction in which water is disassociated to form hydrogen gas; and
- the direct adsorption of a chlorinated hydrocarbon onto the surface of the iron, followed by reductive dehalogenation.
Hydrogen gas can is be used for reductive dehalogenation by the following reaction:
H2 + X-Cl = X-H + H+ + Cl-
This reaction is driven abiotically by the palladium, which acts as a hydrogenation catalyst (palladium can adsorb 900 times its own volume in hydrogen gas). This precise end reaction can also be carried out by the in situ microbial community. Other site specific abiotic processes are also likely to promote the hydrogenation reaction.
Use of the produced hydrogen for hydrogenation of the chlorinated hydrocarbon through catalytic reactions (with palladium or other constituents that may be naturally present in a aquifer) or through biological utilization will improve the stoichiometry of iron consumption. So in addition to the 3 fold improvement in degradation kinetics caused by palladium, the palladium also improves the stoichiometry two fold.
2.1.1.2.2 Oxygen Reactions
A further consideration for iron loading must also take into account that each mole of oxygen dissolved in the groundwater will also consume two moles of metallic iron. An important additional reaction is the reaction of the ferrous iron with oxygen to produce ferric iron hydroxides.
Lastly any oxyanions (from manganese, chromium, arsenic, selenium, molybdenum, etc.) must also be taken into account. Although in most instances, with the exception of sites impacted by chromate, this will be a minor contribution to iron consumption.
3.0 Delivery Modes
In fine grained sediments the colloid can be delivered using hydrofracturing techniques. In permeable granular sediments direct injection of colloids into the pore spaces is possible.
3.1 Fracturing Fine Grained Sediments
In fine-grained soils, with poor fluid transport characteristics, reactive walls could be constructed through hydrofracturing using a proppant that incorporates the reactive colloid. The higher reactivity of the nano scale colloid in conjunction with low overall system flow rates will allow for effective treatment in the confines of a reactive barrier that is only centimeters thick. In contrast, conventional iron filing reactive walls are typically greater then a meter in thickness. Reactive barriers of this type can be installed in or downgradient of a source zone, vertically intersecting the contaminated groundwater flow. Reactive zones are conventionally installed through trenching, where the targeted portion of the aquifer is shallow, and surface improvements do not interfere with access. Using nano scale iron, reactive barriers can also be installed by standard vertical well injection or horizontal borings beneath existing structures if required.
The generation method for vertical fractures is site specific. There is, in most instances, a significant propensity for hydrofractures to have a vertical component that can be exploited for our purposes. The degree to which sediments are consolidated, how the well bore is prepared during pre-injection and the manipulation of weight loads on the surface can enhance the generation of fractures with vertical orientations. Experience has shown that the typical width for induced fractures is the range of 1 cm to 1 inch. The nature of the forces associated with the injection process tend to propagate the fracture rather than increase the width of a fracture. In many terrain’s there would be a tendency for injected colloids to migrate through the walls of the fracture creating a more diffuse wider reaction zone adjacent to the fracture. That phenomena would improve the ability of the reactive wall to perform given the kinetic constraints of the dehalogenation process. In very tight soils (such as tills) the overall migration of groundwater would be slow enough to meet kinetic requirements in such a narrow reaction zone. The nano-scale colloid will generally be reactive enough that the fractures would not be filled with pure colloid, but with a mixture of colloid and sand (to act as a proppant). Pure colloid in a fracture would likely form an impermeable barrier.
3.2 Injection and Stabilization of Colloids in Permeable Sediments
To directly address micro emulsions in source zones of at least moderately permeable material, or to treat dissolved phase material in granular soils, injection of the colloids into the intergranular pore space of the geologic matrix would be the preferable mode of application. Reactive barriers can be created that are vertical to intersect the horizontal flow of dissolved CAHs horizontal to intersect the vertical downward flow of DNAPL. Diffusion of both the colloid generated hydrogen and the CAH to the colloid particles, will provide the intimate contact between micro emulsified/sorbed source material and colloid necessary for effective treatment once the colloid has been dispersed throughout the targeted zone. The injection of colloids into a granular geologic matrix will require some care. The mobility of colloids in the subsurface is governed by mechanical and adsorptive processes.
3.2.1 Mechanical Colloidal Processes
Particles larger than 2 microns, in the low flow conditions common in groundwater systems, are subject to removal by sedimentation (settling under the influence of gravity). Below 0.1 microns the effects of adsorptive process are much more pronounced. As a result, colloids and particles in the range of 0.1 to 2.0 microns are likely to be the most mobile in groundwater. Colloids or other particles can be mechanically removed by the soil matrix. The key parameter to this process is the pore entrance size, which is a function of grain size. For fine to coarse grained silts pore entrance size ranges from 0.7 to 7 microns, for fine to coarse grained sands from 24 to 240 microns, and for fine to coarse grained gravels 720 to 7,200 microns.
Mechanical removal of particles occurs most often by straining, a process in which particles can enter the matrix, but are caught by the smaller pore spaces as it traverses the matrix. If within the soil matrix there is groundwater flow through heterogeneities, a surface mat may form at the interface when particles are too large to enter the finer grained matrix at all. The best example of this is along the walls of fractures through fine grained sediments.
3.2.2 Adsorptive Colloidal Processes
The primary forces that influence the adsorption of colloids to a surface include:
- electrostatic repulsion and attraction;
- London- van der Walls attraction; and
- brownian motion.
Electrostatic forces are familiar. London-van der Walls attraction is a weak (but still effective) form of chemical bonding. Brownian motion is due to molecular collisions between a particle and the surrounding fluid matrix, it becomes apparent when particle size reaches a few microns. The effect predominates in colloids 0.1 microns or smaller, thus the smaller the size the higher the velocity that can be imparted due to brownian motion.
Adsorptive interactions of colloids may be effected by:
- the ionic strength and composition of the groundwater;
- quantity, composition and size of the suspended colloids;
- geologic composition of the soil matrix; and
- flow velocity of the groundwater.
In most instances, the mobility of a colloid is dependent upon solution chemistry rather than forces due to advective flow. Higher mobility occurs at lower overall concentrations of total dissolved solids (TDS), higher levels of which encourage deposition of colloids.
The reasons for this behavior deserve some explanation. Quartz and feldspar surfaces in an aquifer matrix have a net negative charge (above a pH of 3). Therefore, negatively charged colloids are expected to be more mobile than positively charged (the colliod is expected to have a negative charge above a pH of 5 to 6). However, small quantities of other minerals can provide for a localized shift of the point of zero net charge from a pH below 3.0 to approximately pH 8. These mineralogy heterogeneity effects must be taken into account as part of an injection protocol and thus will be investigated during a treatability study.
As the ionic strength of the groundwater increases the thickness of the double layer decreases. When a negatively charged colloid approaches a negatively charged grain within the groundwater matrix (both with double electrical layers) mutually repulsive forces increase. Conversely, if the two surfaces can approach past the repulsive maximum, attractive London-van der Waals forces will take over, overcome the repulsive forces, and the colloid is attached to the matrix surface. The high velocities imparted to colloids smaller than 0.1 microns due to brownian motion provides the mechanism for overcoming the electrostatic repulsion of the double layers. The process is naturally delicately balanced such that the reduction of the electrical double layer thickness through increased ionic strength is also required.
The above issues as well as specific site conditions will govern the specific injection protocols, but in general, the process will have five critical steps:
- Pretreatment of the geologic matrix that will serve as the host for the reactive colloids. This is a process to manipulate a formation that already has a heavy load of existing natural colloidal particles. Flushing of the particles may be required if sites available for the deposition of the iron colloids are previously occupied. Or (more likely) stabilization of those colloids may be required if it is found that injection solutions release massive amounts of natural colloid that plug the aquifer.
- Above ground engineering provisions to prevent agglomeration of the colloid in injection solutions. This will entail control of the ionic state of the suspension fluid to prevent agglomeration, the use of surfactants for the same purpose, and determination of optimum colloid concentration in the solution.
- Prevention of the premature deposition of the colloids during injection into the formation. A portion of this will be controlled by the processes taken in step 2 above.
- Intentional colloid deposition at the desired location in the formation. Not only must the colloids migrate to the desired location, but they must stop when there.
- Treatment to set the colloids in place to prevent future migration. Depending upon the geologic matrix it may be necessary to use a flush with a soluble divalent cation (such as calcium chloride) to irreversibly set the iron colloids onto the surfaces of the geologic matrix.)
4.0 Treatability Studies
The purpose of the bench scale treatability study is to provide site specific design parameters for field application of the technology.
The treatability testing has two phases:
- Evaluation of what will be required to inject the nano-scale colloids into the site subsurface.
- Evaluation of the degradation kinetics using site groundwater and site soils to make a determination of what will the required iron loading be to achieve the clean-up goals for the test at the selected site.
The goals of the treatability laboratory are intended to provide clarify the requirements for technically successful injection and establishment of an in-situ nano scale iron colloid wall. Goals that are supportive of the development of functional injection protocols are:
- To determine whether pretreatment of the geologic matrix at a site is required to facilitate appropriate transport behavior of the injected iron colloids. This will be evaluated by initial X-Ray diffraction (XRD) analysis of the mineralogy of the soil. Subsequent to XRD analysis, column(s) of site soil will be flushed with deionized water (DI). The column elutant will be subjected to testing to identify and quantify inorganic dissolved and colloidal species.
- To design the colloidal suspension capable of preventing particle agglomeration during make-up and injection. This will involve jar testing of nano-scale colloids with soluble solution enhancements known to be useful in the prevention of particle agglomeration.
- To investigate the chemical mechanisms controlling colloid deposition in the geologic matrix it is important to understand both the natural colloid deposition characteristics and how to favorably influence the natural tendencies to achieve a suitable zone of influence for each injection well.
- If required by the site geology, a determination of treatment to prevent future migration of the colloids following injection will be performed. The specific mechanism to be evaluated during this treatability task is flushing the geologic matrix in colloid loaded column(s) with a soluble divalent. The effectiveness of this approach will be determined by monitoring column elutant for the colloid.
During the treatability study, reaction kinetics will be evaluated using columns. Each column will be operated at a different iron loading. One column will be conservative assuming almost instantaneous reaction and thus a short required contact time between the CAHs and the colloid. The second column will be designed to evaluate the effect of lower concentrations of iron loading compensated by a longer reaction time with the reactive zone.
Return to the Environmental Technology Index Page
Copyright 2002 David B. Vance
All Rights Reserved![]()
If you have comments or suggestions, e-mail me at dbv7@mindspring.com