|
2 The 4 Technology Solutions

REMEDIATION BY IN-SITU AERATION
THE POWER OF VOLATILIZATION AND BIO-OXIDATION
An On-Line Version of an Article First Published in:
The National Environmental Journal July/Aug. 1993 Vol. 3 No. 4 Pages 59-62
By: David B. Vance and Howard Schuener
|Soil Vapor Extraction | Bio-Oxidation | SVE vs. Bio-Oxidation | Biosparging Example | References|
dbv7@mindspring.com
INTRODUCTION
Soil venting is a term used for an in-situ aeration process that is a powerful remediation technology for the treatment of soils exposed to a variety of hydrocarbons. The practice of soil venting includes the following variations in application:
- Soil vapor extraction systems are designed to exploit a hydrocarbons potential for volatilization.
- Air sparging is an aeration process in which volatilizing air is injected into the saturated zone beneath the water table. Soil venting is used to recover the vapor laden air as it exits the water table.
- Bioventing is an aeration process designed to deliver oxygen to the subsurface for use by indigenous bacteria to degrade hydrocarbons, the focus is on minimizing hydrocarbon volatilization.
- Biosparging is a variant of air sparging where oxygen stimulated biodegradation is the aim, rather than volatilization. As with air sparging, soil venting is used to recover gas discharged through the water table.
Figure 1 illustrates and compares the potential effectiveness of soil vapor extraction and bioventing.
The data illustrated in Figure 1 was calculated using the following premises:
- The air flow rate is 10 SCFM (Standard Cubic Feet per Minute).
- For the vapor transport calculations, it is assumed that the 10 SCFM air stream becomes saturated with hydrocarbon vapor.
- Volatilization driving vapor pressures were calculated at 8o centigrade.
- For the biodegradation calculations, it is assumed that the oxygen provided by the 10 CFM air flow is completely utilized for hydrocarbon bio-oxidation.
- Biodegradation of each of the hydrocarbons proceeded to carbon dioxide and water.
Figure 1, Hydrocarbon Removal by SVE and Bio-Oxidation
This data was derived from theoretical calculations predicated on the fundamental principals governing the action of each of the processes (i.e. volatilization or bio-oxidation). In actuality, these processes and other subsurface interactions (with soil moisture for example) are more complex. However, the base principals do apply and are, in the overall process, upheld. Although based on a simplified system, the data serves to illustrate the fundamental principals of, and differences between, soil vapor extraction and bioventing.
Following is a more detailed explanation of these processes and a case history that illustrates the use of biosparging technology at a site contaminated with volatile and non-volatile hydrocarbons.
SOIL VAPOR EXTRACTION
By stimulating an advective air flow through soil venting, adsorbed hydrocarbons are exposed to air that does not carry an existing vapor load. Upon this exposure, equilibrium driven mechanisms will force the hydrocarbons to volatilize into the induced air flow. Every hydrocarbon has a specific temperature dependent vapor pressure that determines the maximum (saturated) vapor concentration that can be obtained in the air flow. This vapor saturated air is directed to the surface where it may be discharged to the atmosphere, or more commonly, treated to remove the hydrocarbon vapors before discharge.
The key element in this vapor extraction process is the fact that the mass transport rates are determined by a physical property (the vapor pressure) of the hydrocarbon. Not all hydrocarbons have the same vapor pressure, and the lower the vapor pressure, the lower the overall mass transport rate will be for that compound. Thus, the dramatic trend seen in Figure 1 for the SVE portion of the graph. Table 1 presents the basic data used to prepare the soil vapor extraction part of Figure 1.
Table 1, Vapor Pressure and Volatilization Driven Mass Transport Rate
|
Hydrocarbon
|
Vapor Pressure*
|
Molecular Weight
|
Saturated Vapor Concentration
|
Lbs Removed in One Day at 10 SCFM
|
|
Benzene
|
41.0
|
78.1
|
11.5 x 10-3 lbs/ft3
|
166
|
|
Toluene
|
11.1
|
92.1
|
3.67 x 10-3 lbs/ft3
|
52.8
|
|
Ethylbenzene
|
5.5
|
106.2
|
2.10 x 10-3 lbs/ft3
|
30.2
|
|
Xylene
|
4.0
|
106.2
|
1.53 x 10-3 lbs/ft3
|
22.0
|
|
Naphthalene
|
0.02
|
128.2
|
9.2 x 10-6 lbs/ft3
|
0.13
|
* mm Hg at 8o Centigrade
Benzene is extremely volatile and offers an excellent mass transport potential of 166 pounds per day. However, naphthalene is at the other extreme, with a vapor pressure less than 1 mm Hg, it is at the lower end of hydrocarbons considered amenable to removal by soil vapor extraction. In this instance only 0.13 pounds per day would be removed. The other petroleum hydrocarbons fall somewhere between the two.
The dependance of soil vapor extraction on the varying physical property of vapor pressure places serious constraints on the rate of SVE remediation at sites impacted with petroleum hydrocarbons. Even gasoline has significant concentrations of hydrocarbons with relatively low vapor pressures. Heavier petroleum products such as jet fuel, kerosene, diesel fuel and lubricating oil are thought to be non-responsive to soil vapor extraction technology. Based on soil vapor extraction alone, that thought is an accurate one.
Air sparging is the process of hydrocarbon volatilization stimulated by the injection of air beneath the water table. With air sparging the volatilization process takes place under saturated conditions, emulating the action of an air stripping surface treatment system. The governing physical parameter that relates a hydrocarbons volatilization potential from water is the Henry's constant. Which is directly related to a hydrocarbons vapor pressure, water solubility and temperature of the air/water/hydrocarbon system (Haarhoff and Cleasby, 1990). This is a more complex physical/chemical system than volatilization of free phase or adsorbed hydrocarbons from soil in a vapor extraction system. In an air sparging system the injected air and entrained volatilized hydrocarbons are captured above the water table with a conventional soil venting system.
BIOVENTING
Bioventing is the term for aerobic biodegradation stimulated by oxygen provided to a hydrocarbon impacted subsurface zone by an air flow induced through soil venting. The power of bioventing lies in underlying process responsible for it, the biologically mediated oxidation of hydrocarbons to carbon dioxide and water.
The data calculated for the bioventing portion of Figure 1 is based on the stoichiometric consumption of oxygen. That is, each mole of carbon present will require one mole of oxygen (O2) to be converted to carbon dioxide (CO2):
O2 + C = CO2
Each mole of hydrogen requires 1/4 mole of oxygen (as an O2 molecule) to produce one mole of water. It can be more conveniently expressed as follows:
O2 + 4 H 2 = 2H2O
The exact stoichiometry for each of the hydrocarbons illustrated in Figure 1 is as follows:
Table 2, Bio-Oxidation Stoichiometry
Compound Chemical Formula Moles O2 Required Oxidation Products
Benzene C6H6 + 7.5 O2 = 6 CO2 + 3 H2O
Toluene C5H5(CH3) + 9 O2 = 7 CO2 + 4 H2O
Ethylbenzene C6H5(C2H5) + 10.5 O2 = 8 CO2 + 5 H2O
3-Xylene C6H4(CH3)2 + 10.5 O2 = 8 CO2 + 5 H2O
Naphthalene C10H8 + 12 O2 = 10 CO2 + 4 H2O
A flow rate of 10 SCFM can deliver 270 pounds of oxygen to a treatment zone in a period of 24 hours. Based on the above stoichiometry, the potential biodegradation rates in pounds per day are as follows:
Table 3, Bio-Oxidation Rate
Compound Bio-Oxidation Potential - Lbs/Day
Benzene 88
Toluene 87
Ethylbenzene 86
3-Xylene 86
Naphthalene 90
This entire process is independent of any other physical property of these hydrocarbons. The prime issue with regards to in-situ remediation by bioventing is how much oxygen can effectively be transported to the reaction (contaminated) zone.
However, there are practical limits to the effectiveness of biodegradation and the linear effect of the stoichiometric bio-oxidation reaction with oxygen. The potential problem lies with compounds that are recalcitrant to biodegradation. With respect to petroleum hydrocarbons, these recalcitrant compounds are typically polynuclear aromatic (PNA) compounds having high ring counts. In many products, however, high ring count PNAs are not a significant amount of the total hydrocarbon makeup. In addition, these compounds are most often still biodegradable, but at a slower rate than that observed for the less refractory hydrocarbons.
Indigenous Bacteria
A key concern over the viability of the bioventing approach is the presence of indigenous bacteria capable of being stimulated to degrade hydrocarbons. Figure 2 illustrates soil gas data from a site impacted with petroleum hydrocarbons.
Figure 2, Bioactive Soil Gases in the Vadose Zone
These samples were collected under static conditions, no remediation activity has taken place. This soil gas is representative of equilibrium conditions existing at the impacted site.
Although volatilized hydrocarbon vapors (from the contaminant impact) are also present in the soil gas, Figure 2 only shows the three dominant soil gases: carbon dioxide, oxygen and methane.
Under normal atmospheric conditions oxygen concentration is 21 percent. Carbon dioxide is approximately 300 ppm (0.03%). Due to the presence of carbonate minerals and natural organic materials, CO2 in uncontaminated soils is typically found at concentrations from 1.5 to 3% (Suchomel et al, 1990). Methane is present in the atmosphere in trace amounts (1.5 ppm). As Figure 2 illustrates, the soil gas concentrations at this contaminated site are significantly skewed from those levels. Carbon dioxide is elevated at 11% to 12%, oxygen is depressed to around 5% and methane is elevated at 3% to 5%.
Following is an outline of the process responsible for the generation of this soil gas blend:
- Upon release of hydrocarbons into the subsurface the indigenous bacteria began an aerobically driven bio-oxidization process.
- The end product of this aerobic microbial degradation was carbon dioxide and water (see Table 2).
- After the aerobic microbial activity had consumed oxygen in the soil gas to near the observed 5% level, facultative anaerobes became active. These bacteria have the ability to support metabolic activity under full aerobic or oxygen depressed conditions (the transition has been observed in the field and laboratories to normally occur at oxygen levels near 5%).
- The degradation products (seen in the gas phase) of the anaerobic activity are methane and additional carbon dioxide.
The fundamentally important point of the data illustrated in Figure 2 is that this aerobic/anaerobic activity occurred naturally. There were no bacteria added to the soil, the existing indigenous bacteria generated these gases. There were no nutrients added. The indigenous bacteria were able to become active under natural subsurface conditions, using available: oxygen; nitrogen; phosphorous; and trace nutrients. With depletion of the oxygen levels the facultative anaerobic activity became dominant at the expense of the aerobic bio-oxidation. This anaerobic degradation occurs at a rate several orders of magnitude slower than that observed for aerobic degradation (Atlas, R.M., 1981). If this were not the case, it would be cost effective to let the anaerobic degradation occur at its own pace with no other intervention.
What is required for timely bioremediation is the installation of a soil venting system to displace this soil gas (equilibrated to the existing chemical and microbiological conditions) with fresh, fully oxygenated air. Thus, re-stimulating the natural aerobic biodegradation of the impacting hydrocarbons. This is the essence of the bioventing process.
The governing processes engaged in a biosparging system are identical to those in bioventing. The biosparging stimulated bio-oxidation follows the same stoichiometry presented in Table 2. The injected air, bio-oxidation products (CO2) and some fraction of volatilized hydrocarbons are collected above the water table with a soil venting system, just as described for air sparging.
The last sentence contained a key statement, that "some fraction of volatilize hydrocarbons" are generated by a biosparging (and bioventing) system. The next section analyzes this in more detail.
SOIL VAPOR EXTRACTION VERSUS BIOVENTING
A SIMULTANEOUS PROCESS
When a soil venting system is operated at a site impacted by petroleum hydrocarbons both of the discussed mechanisms are engaged. The determination of which process is dominant lies in how the soil venting system is operated.
A soil venting system can be operated such that 60 to 90 percent of the impacting hydrocarbons are volatilized and 10 to 40 percent are biodegraded, i.e. soil vapor extraction. Bioventing reverses those numbers, 60 to 90 percent of the hydrocarbons are biodegraded and 10 to 40 percent are volatilized (Miller et al, 1990). Note that some degree of biodegradation is unavoidable even in a system that is designed and operated as a pure soil vapor extraction system. The site soils would have to be sterilized to prevent it. Conversely, some level of volatilization is equally unavoidable in a bioventing system.
The value of the bioventing approach lies in three areas:
- First, every pound of hydrocarbon that is degraded in-situ in the subsurface is a pound that will not require subsequent treatment on the surface, resulting is significant cost savings.
- Equipment costs and operational costs (primarily energy) are less using the lower air flow rates capable of supporting the bioventing approach. Oxygen does not need to be brought to the subsurface at a rate faster than the ability of the bacteria to consume it.
- Third as Figure 1 illustrates, soil vapor extraction does not work well with hydrocarbons that have low vapor pressures.
Remediation by soil vapor extraction is very rapid when applied to hydrocarbons with high vapor pressures. For example, a release of pure benzene could theoretically be cleaned up faster using a straight soil vapor extraction approach rather than bioventing. Although, surface treatment expenses must still be taken into account in the overall project costs.
Soil vapor extraction is definitely the preferred approach when remediating biological recalcitrant compounds that have high vapor pressures such as chlorinated solvents. However, in instances where the released materials are petroleum hydrocarbons such as fuels or lubricants, bioventing is likely to the be most cost and time effective remediation approach.
BIOSPARGING - A CASE HISTORY
Following is a field example of the biosparging process discussed above. This project is on-going at a facility which was closing a RCRA hazardous waste drum storage area (DSA). The wastes stored in the DSA were "Spent Non-Halogenated Solvents" (F003/F005). Figure 3 shows a plan view of the site, the DSA, and the installed remediation system.
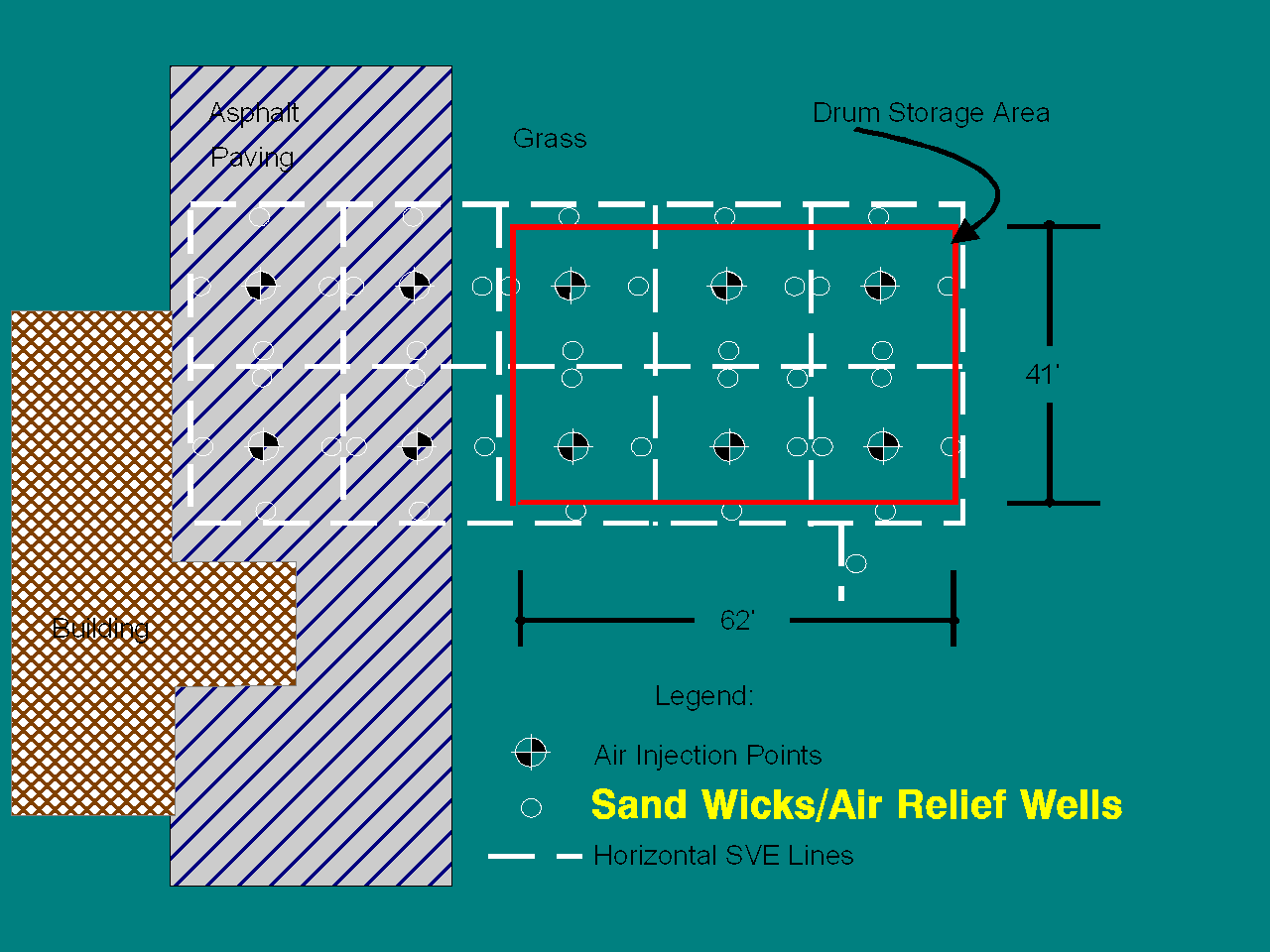
Figure 3, Plan View of Biosparging Site
The geology beneath the site consists of:
- approximately 30 feet of sand inter bedded with a peat layer 1 to 3 feet thick at a depth of 16 feet. Silt and clay underlies the sand.
- Depth to groundwater is 6 feet below grade (see Figure 4).
- Soil and groundwater contamination was limited to the sand/peat unit above the clay.
- Soil contamination included: ethylbenzene (160 mg/Kg); toluene (110 mg/Kg); total xylenes (620 mg/Kg); naphthalene (440 mg/Kg) and other polycyclic aromatics at low mg/Kg levels.
- Groundwater contained 2 mg/L ethylbenzene and 16 mg/L total xylenes.
The remediation system put into place was an in-situ saturated zone treatment using biosparging. The details (Figure 4) of which are as follows:
- A series of sparge points were installed to a depth of 30 feet just above the surface of the lower clay layer. This placed them about 14 feet beneath the peat layer.
- A series of 4 inch borings, filled with graded sand to act as air relief wells (sand wicks), were installed to a depth of 30 feet. The sand wicks act as a conduit for injected air bubbles through the peat layer, preventing unacceptable horizontal migration of the injected air and any entrained vapor. This is a common and critical problem for the application of air sparging/biosparging in heterogeneous soils.
- A soil venting recovery system was installed in horizontal trenches above the water table to capture the injected air and hydrocarbon vapors generated from the biosparging system. The area was then covered with a plastic liner and soil to prevent short circuits in the soil venting system.
- The discharge of the soil venting system was treated with vapor phase activated carbon.
 |
Figure 4, Cross Sectional View of Biosparging Site with Sand Wicks
An important point with regards to this system was the biosparging aspect of the design. While vapor phase activated carbon was installed as a necessary treatment of co-produced hydrocarbon vapors, the intent of the remedial design was to minimize the actual volatilization and concentrate on oxygen stimulated biodegradation of the hydrocarbons in-situ. This approach minimized the carbon usage and also had the potential to remove the non-volatile constituents of the impacting hydrocarbons. To this end, air injection was only at 12 SCFM, a rate estimated to match the kinetics of the microbiological bio-oxidation. The horizontal soil venting system was operated at 60 SCFM to insure that all the injected air (and minimal hydrocarbon vapor load) were adequately recovered.
While provisions were made for the addition of nutrients (nitrogen, phosphorus and trace minerals), nutrients were not actually utilized in this phase of the project. Under many conditions existing nutrient levels are often adequate to support microbial activity at an acceptable level. Under most circumstances oxygen supplementation is the dominant factor required for microbial stimulation. Nutrient addition to the vadose and saturated zones (while possible) is a complication that should be avoided unless required.
Figures 5 and 6 illustrate the results after system start-up. Figure 5 illustrates the trend of the vaporized hydrocarbon and carbon dioxide observed in the recovered soil gas and Figure 6 illustrates the trend of dissolved oxygen (DO) in the groundwater and carbon dioxide in the soil gas.
Figure 5 shows a sharp increase in the concentration in the VOCs recovered in the soil gas by day 10 of system operation. Initial carbon dioxide levels are quite low. In a manner very typical of microbiological systems, carbon dioxide concentrations steadily increased to a maximum level over a
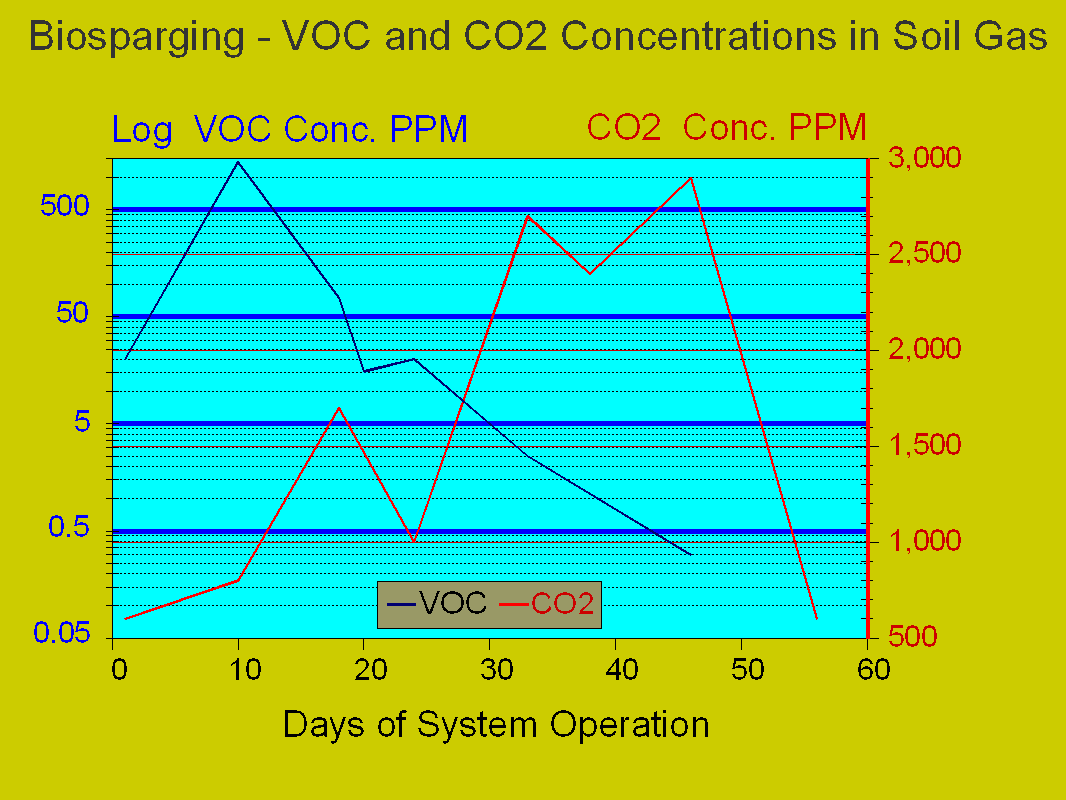
Figure 5, Hydrocarbon Vs. Carbon Dioxide in Soil Gas Above Biosparging
30 day period. This period is termed the "lag phase", as the indigenous microorganisms adapt to the sudden introduction of oxygen into their environment and the utilization of the impacting hydrocarbons as a carbon source.
In turn, the VOC concentrations continue a steady decline as degradation begins to become more dominant. The most striking feature of this data is the continuous generation of significant amounts of carbon dioxide after day 30, with very low concurrent levels of VOC emission. This is due primarily to the bio-oxidation of nonvolatile hydrocarbon components (such as lighter PNAs), which are not responsive to removal through volatilization.
Lastly, after 50 days of operation the carbon dioxide levels precipitously decline, in conjunction with VOCs becoming almost undetectable. This indicates that the hydrocarbons have been consumed and the stimulated bacteria have ran out of the hydrocarbons serving as their carbon source.
Figure 6 serves to further illustrate the biological activity in the saturated zone of the impacted soils. The initial low levels of the dissolved oxygen may have been to due to chemical oxygen demand within the aquifer matrix (primarily dissolved iron). After meeting that COD, the DO increased in tandem with carbon dioxide production.
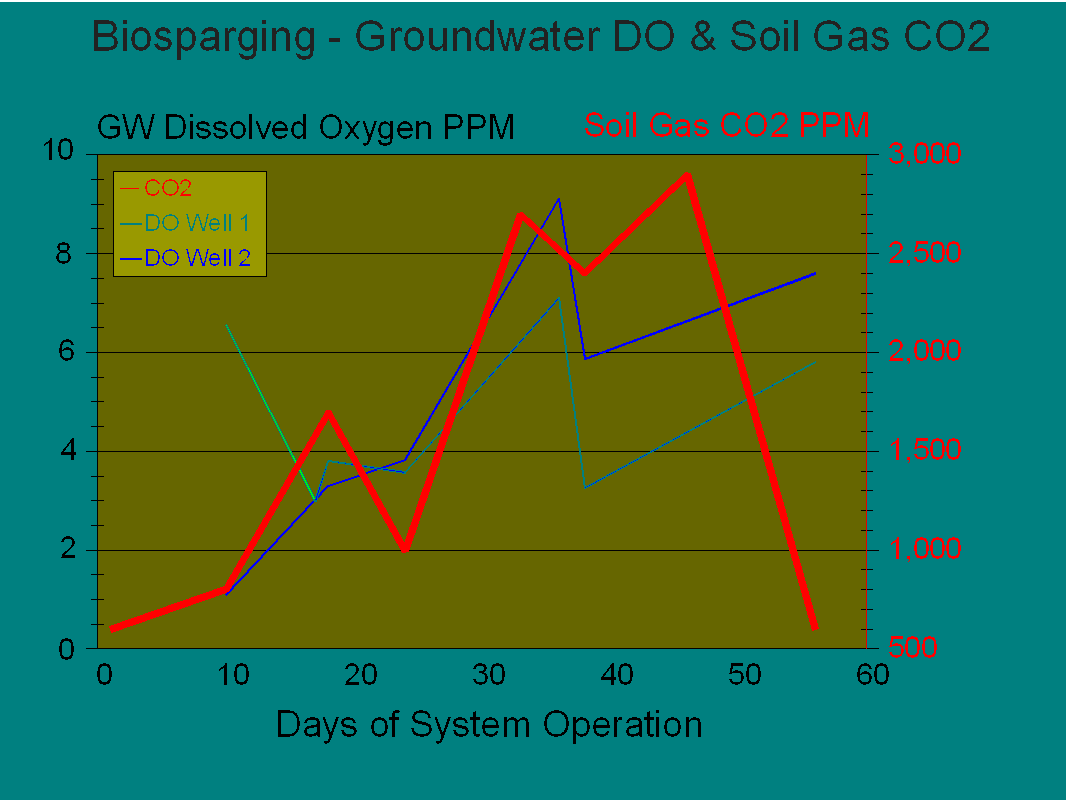
Figure 6, CO2 in Soil Gas Vs. Dissolved Oxygen in Groundwater
Of particular interest is the marked drop in DO between day 35 to 40. This coincides with the peak biological activity. When the carbon dioxide levels began to drop the DO increased once again. At the peak level of activity the microbes were able to consume most of the oxygen provided by the injected air, as planned. With depletion of the food source, more oxygen is free to appear as DO.
This site data has been presented to provide a topical field example of biosparging. It is from the early stages of the remediation. The hydrocarbon attenuation indicated by Figures 5 and 6 is occurring in the advective zone of the impacted saturated zone soils and groundwater. What still must be addressed are adsorbed hydrocarbons associated with low permeability diffusional transport zones and with the peat layer (Vance, D., 1993 a and b). This portion of the site remediation is still in progress.
In conclusion, aeration is a powerful remediation tool in the vadose and saturated zones. The manner in which an aeration system is operated will determine the dominant process stimulated by that aeration; volatilization or bio-oxidation. Bio-oxidation has distinct advantages with regards to the range of non-volatile hydrocarbons that can be remediated and offers lower potential off-gas treatment costs. The core issue is to understand the underlying mechanisms responsible for both processes and utilize that knowledge accordingly.
CITED REFERENCES
Atlas, R.M., 1981. Microbial Degradation of Petroleum Hydrocarbons: an Environmental Perspective, Microbiological Reviews, Vol. 45, No. 1, pp. 180-209.
Haarhoff, J. and Cleasby, J.L., 1990. Evaluation of Air Stripping for the Removal of Organic Drinking-Water Contaminants, Water SA, Vol. 16, No. 1, pp. 13-22.
Miller, R.N., Hinchee, R.E., Vogel, C.M., Dupount, R.R., and Downey, D.D., 1990. A Field Scale Investigation of Enhanced Petroleum Hydrocarbon Biodegradation in the Vadose Zone at Tyndall AFB, Florida, Proceedings: NWWA Petroleum Hydrocarbons and Organic Chemicals in Ground Water Conference, Houston, Texas, pp. 339-351.
Suchomel, Karen Hohe, Kreamer, David K. and Long, Austin, 1990. Production and Transport of Carbon Dioxide in a Contaminated Vadose Zone: A Stable and Radioactive Carbon Isotope Study, Environ. Sci. Technol., Vol 24, pp. 1824-1831.
Vance, David B., 1993 A. Groundwater Remediation - First Principals, The National Environmental Journal, Vol. 3, No. 2, pp. 18-20.
Vance, David B., 1993 B. Hydrophobic Organic Chemicals and Total Organic Carbon - The Ideal and Reality of Site Clean-Up, The National Environmental Journal, Vol. 3, No. 3..
Return to the Environmental Technology Page
Return to the 2 The 4 Technology Solutions Home Page
Copyright 2016 David B. Vance
All Rights Reserved

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
|