|
2 The 4 Technology Solutions

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
REACTIVE BARRIERS TO TREAT AREAS ADJACENT TO SOURCE ZONE
An On-Line Version of a Column First Published in:
Environmental Technology July/Aug. 1997 Vol. 7 No. 4 Pages 16-18
by: David B. Vance dbv7@mindspring.com
Much attention has been given to natural attenuation (1),(2),(3), (4) as a cost effective means to achieve risk based clean-up goals for dilute distal portions of contaminant plumes. However, natural attenuation processes are not applicable to source zones nor to areas with high levels of adsorbed contaminants adjacent to them. The usual remediation method for a source zone is excavation, followed by off-site disposal. Pump and treat is typically applied to adjacent soils and groundwater that are not economic to excavate. However, this column will discuss a more passive approach than pump and treat, the use of reactive barriers.
A reactive barrier is installed downgradient of a source zone, vertically intersecting the contaminated groundwater flow. A reactive barrier can be installed with trenching where the targeted portion of the aquifer is shallow and surface improvements do not interfere with access. A reactive barrier can also be installed by well injection. Injection through standard vertical wells is the least expensive when practical, but horizontal borings can be installed beneath existing structures and offer the ability to create a uniform reactive zone that is more difficult to achieve with vertical injection wells.
The reactions that may be stimulated within a barrier fall into three major categories: physical; chemical; and biological. Selection is dependent upon: the type, physical/chemical character, and concentration of the contaminant(s); the hydrogeologic setting of the site; and the regulatory clean-up goals. Contaminants fall into two major categories, inorganic and organic. Inorganic contaminants are usually immobilized. Organic compounds can immobilized, mobilized, or destroyed.
Immobilization mechanisms for the treatment of inorganics include precipitation, adsorption, and other chemical changes into insoluble forms. Any immobilization technology is going to increase mass/volume within the pore space of the reactive zone. A concern, particularly with injection based systems is blockage of the pore space with products of the reaction processes.
A standard scenario for this and following comparisons is a reactive barrier with the following dimensions: 10 feet deep, 3 feet wide and 100 feet long; assume 25% porosity for a barrier created by injection and 50% porosity for a barrier constructed by trenching; further assume that only 25% of available porosity can be utilized for storage of reaction products without adversely affecting flow through the barrier. This leaves about 14 cubic yards of space available for the deposition of reaction products in the trench and 7 in the injection barrier.
To compare treatment capacity, assume the following as a standard scenario for a contaminant plume: dimensions are 100 feet by 100 feet and 10 feet in thickness, with 50% aquifer porosity. Metal treatment capacity of a reactive barrier configuration is expressed as a metal concentration in that plume (i.e. 50 mg/L, means the reactive barrier configuration could treat a plume of the above dimensions with 50 mg/L of dissolved metal contaminant.)
For precipitation reactions the treatment capacity is 30 mg/L for the trench and half that for the injection barrier. Unfortunately in most instances, the pH of the groundwater discharging from a precipitation barrier will be above the allowable regulatory limits.
Removal of metals through biological action can be an attractive, particularly with injection barrier systems. Microbes are used to create the appropriate redox conditions, in conjunction with the supply of nutrients/chemicals that the bacteria use to mediate metal immobilization reactions. The most practical process would use sulfates to convert the metals into insoluble sulfides. Compared to pH precipitation, the solid phase that would occupy a volume 5 to 10 times less (sulfides are dense), increasing treatment capacity to 150 to 300 mg/L (using the standard scenarios described above.) Provisions may be required to raise the redox state of the discharging groundwater, but this is an easier process than the drastic pH reduction required of an alkaline precipitation water.
Lastly, metals can be removed by adsorption. Ferric iron hydroxide systems are particularly attractive. Ferric hydroxide has the capacity to adsorb a wide variety of metals in cationic or anionic form in pH ranges normal to groundwater and the ferric hydroxide adsorption media will not dissolve into the groundwater at that pH. Although, accurate contaminant assessment and pH control will still be required because removal efficiencies typically occur within a narrow band (i.e. pH 6.8 versus. pH 7.4 can make a significant difference in residual contaminant concentration.) Standard scenario treatment capacities are in the 80 to 40 mg/L range.
Hydrocarbon treatment with a reactive barrier is extremely viable. This is due to the flexibility of microbial degradation systems that can be stimulated in the barrier. Arrangements for the injection of air, nutrients, co-metabolites, and other chemical supplements is technically straightforward. Redox conditions can be controlled, as well as stimulation of the specific microbial enzymatic systems required to degrade xenobiotic hydrocarbons. The most critical issue is control over the generation of biomass. Over-growth will destroy the hydraulic permeability of the barrier.
Reductive dehalogenation of chlorinated solvents with metal reduction systems (such as metallic iron) can be performed in reactive barrier trenches (with microbes used to maintained desirable redox conditions). Air injection can be used in a straight forward fashion as an in-situ air stripping treatment, with vapor recovery installed above the groundwater table. Lastly, electrochemical cells can be installed and operated to remediate metal and hydrocarbon containing groundwater.
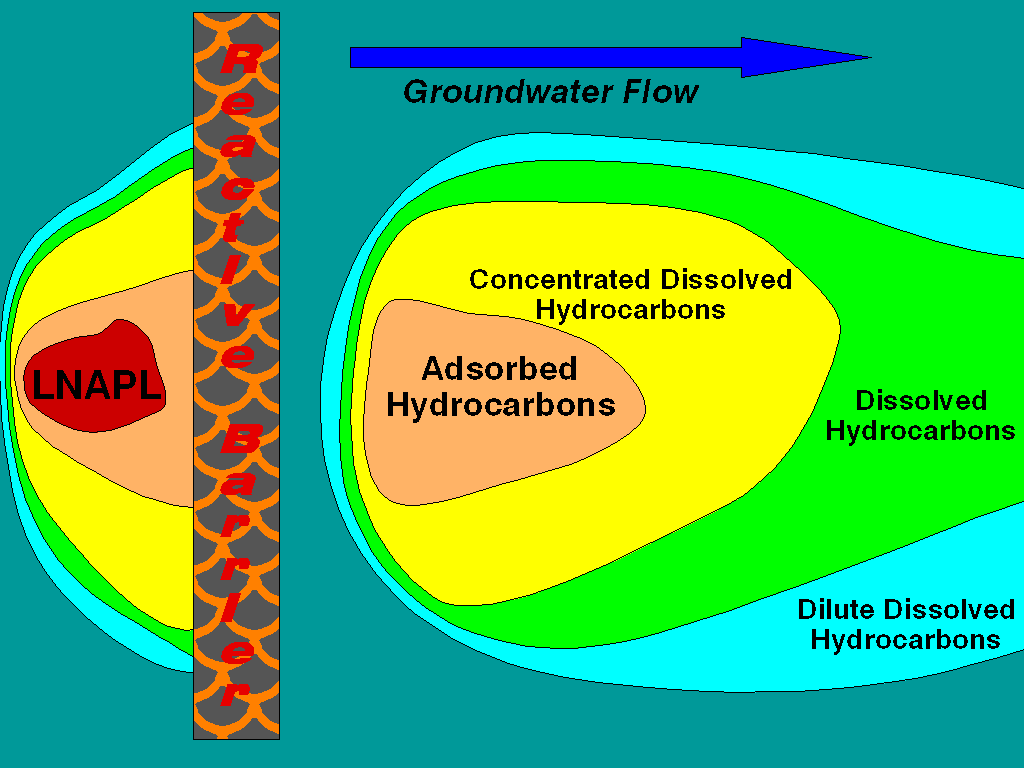
Reactive barriers offer an in-situ treatment of groundwater emitting from source zones. Installation costs may or may not be greater than pump and treat systems, although costs for down-hole and surface equipment are much less. Each recovery well in a typical pump and treat system is going to require $10,000 to $15,000 worth of pumps, support equipment and development costs that are not required for a reactive barrier system. In addition, the installation of a modest pump and treat system will require $20,000 to $80,000 for a surface groundwater treatment system. Operational costs for a reactive barrier will be 90 to 70 percent less (depending upon the scale of analytical monitoring required) than the cost of a pump and treat system per year because no provision must be made for the disposal of recovered water and the system is mechanically passive. The driving force for groundwater through the treatment system is the natural hydraulic gradient at the site. Lastly, a system of this type can exploit the reactive nature of the groundwater discharged from the reactive barrier to enhance natural attenuation rates downgradient of the barrier. That is the scenario illustrated in the figure.
Return to the Environmental Technology Index Page
Copyright 2008 David B. Vance
All Rights Reserved

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
|