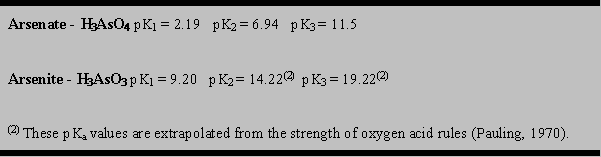2 The 4 Technology Solutions
![]()
ARSENIC - CHEMICAL BEHAVIOR AND TREATMENT
An On-Line Version of an Article First Published in the:
National Environmental Journal 1995 Vol. 5 No. 3
By: David B. Vance
|Chemical Character | Behavior in Groundwater | Behavior in Water Treatment Systems | Conclusions |
I. INTRODUCTION
Arsenic is not an abundant element in the earth's crust, the average crustal concentration of 1.8 mg/Kg ranks it fifty-two, between tin and molybdenum (Demayo, 1985). However through geogenic processing of crustal materials, arsenic can be concentrated in soils to a typical range of 2 to 20 mg/Kg, with concentrations as high as 70 mg/Kg unremarkable (Yan-Chu,1994). Human activity generates anthropogenic arsenic, making it the third most common regulated inorganic contaminant found at CERCLA sites. Arsenical copper was in use by 4,000 BC and the toxic effects of arsenic were documented by early Greek writers. Modern usage of arsenic includes formulation of pesticides and herbicides, decolorization of glass, treatment/preservation of wood, paint manufacturing, and the production of semiconductors. Arsenic is troublesome concerning mobility and treatment. Definition of why that is so is the focus here, particularly of geogenic arsenic in groundwater supplies.
Arsenic may occur as a semi metallic element (Aso), arsenate (As5+), arsenite (As3+), or arsine (As3- ). The chemical character of arsenic is dominated by the fact that it is labile, readily changing oxidation state or chemical form through chemical or biological reactions that are common in the environment. Therefore rather than solubility equilibrium controlling the mobility of arsenic, it is usually controlled by redox conditions, pH, biological activity, and adsorption/desorption reactions. Arsenic in groundwater most often occurs from geogenic sources, although anthropogenic arsenic pollution does occur. Geogenic arsenic is almost exclusively as arsenite or arsenate. Anthropogenic arsenic may have any form including organic arsine species. Groundwater in acidic to intermediate volcanic rocks, or in sediments derived from those rocks, will often have arsenic concentrations exceeding 10 µg/L (the current drinking water standard).
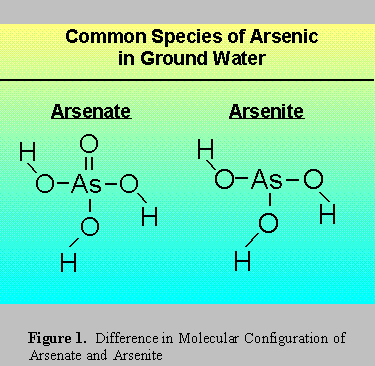
Figure 1 illustrates the difference in molecular structure between arsenate and arsenite. The double bond oxygen in the arsenate molecule influences its ability to become ionized through the loss of
hydrogen ions, the process is termed dissociation. A negative charge develops on the molecule when dissociation occurs. The double bond oxygen increases the capacity to delocalize that charge, easing the loss of hydrogen ions. The propensity for ionization is expressed by pKa the constant of dissociation (which is a negative log, a smaller number shows a greater degree of dissociation).
For arsenate and arsenite pKa values are as follows:
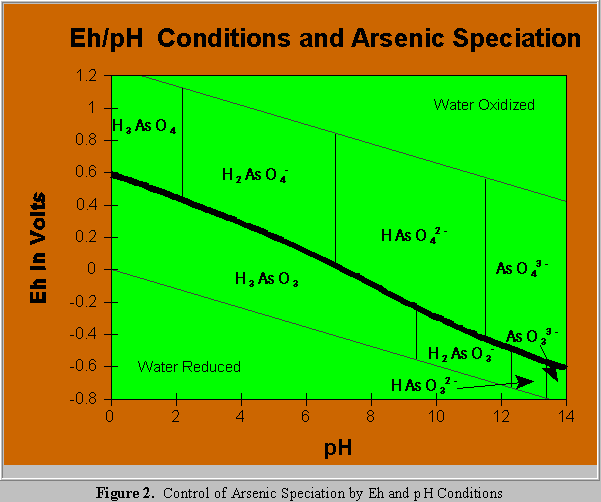
The pH at which these ionization steps occur is significantly different between arsenate and arsenite, as illustrated in Figure 2 (after Robins,1985 and Welch, 1988). Figure 2 also shows the control of
redox potential (Eh) on the arsenate/arsenite transition. This Eh/pH relationship is key in understanding arsenic mobility in groundwater and the effectiveness of arsenic water treatment systems.
III. ARSENIC IMMOBILIZATION
The previous section described the conditions under which arsenic can become an ionized species. The most commonly recognized adsorption reactions are based on ion exchange between charged adsorption sites and charged soluble ions. However, another mechanism is also responsible for adsorption, London Van der Waals bonding. This type of bonding is the result of complex interactions between the electron clouds of molecules, molecular polarity, and attractive forces of an atomic nucleus for electrons beyond its own electron cloud. Consequently some degree of immobilization can occur with soluble species that are not ionized. Arsenic immobilization through ionic adsorption can be controlled within normal oxidizing Eh/pH conditions. London Vander Waals bonding is complex to the point of unpredictability except for arsenic mobility at extreme Eh/pH conditions that can be obtained in industrial settings, but not in groundwater.
Components of soil that participates in both types of adsorptive reactions include clays, carbonaceous material, and oxides of iron, aluminum, and manganese. In the upper reaches of the soil column organic fractions typically dominate, while at greater depths iron oxyhydroxides play the principal adsorptive role.
The typical iron content of soil ranges from 0.5% and 5%. Not only is iron common, but as with arsenic, it is also labile and readily reflects changes in surrounding Eh/pH conditions. This relationship for iron is illustrated in Figure 3 (after Hem, 1961).
Ferric hydroxide acts as an amphoteric ion exchanger. Depending on pH conditions, it has the capacity for cation or anion exchange. Given the average iron concentration in soil and soluble arsenic concentrations in groundwater at 50 µg/L, ferric hydroxides in sediment can potentially adsorb 0.5 to 5 pounds of arsenic per cubic yard of aquifer matrix. This may then act as a significant potential reservoir for arsenic release under changing Eh/pH conditions.
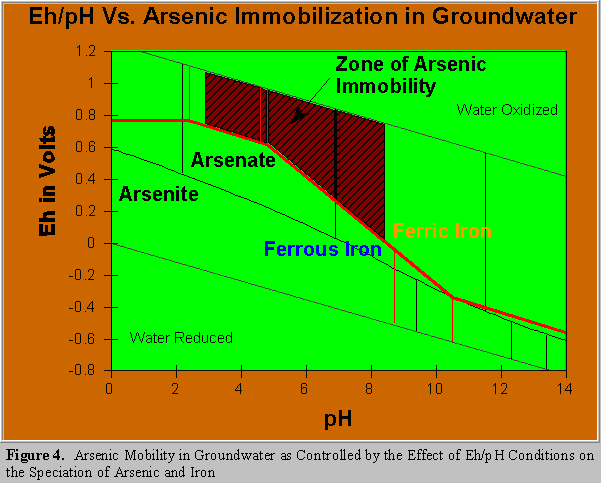
Figure 4 superimposes the Eh/pH relationship for the arsenic and iron systems, it illustrates the conditions under which arsenic will be immobilized in a groundwater system. Of equal importance it illustrates how arsenic adsorbed to ferric hydroxides in sediment can be released upon exposure to
groundwater that is chemically reducing. Two effects would be at work, arsenate is reduced to arsenite that will not remain ionically bound to the geologic substrate and ferric iron is reduced to ferrous, which is soluble under normal pH conditions. Outside the immobilized zone arsenic mobility is variable. London Van der Waals bonding of arsenite is in effect, but it is not sufficient to assure complete immobilization.
IV. WATER TREATMENT SYSTEMS
INTRODUCTION
Following is a brief review of various technologies used for the removal of arsenic from drinking water and industrial wastewater. Table 1 summarizes the effectiveness of each and gives the source for the information.
Table 1. Effectiveness of Arsenic Water Treatment Methods
|
*The current limit for drinking water is 50 µg/L
ARSENITE OXIDATION
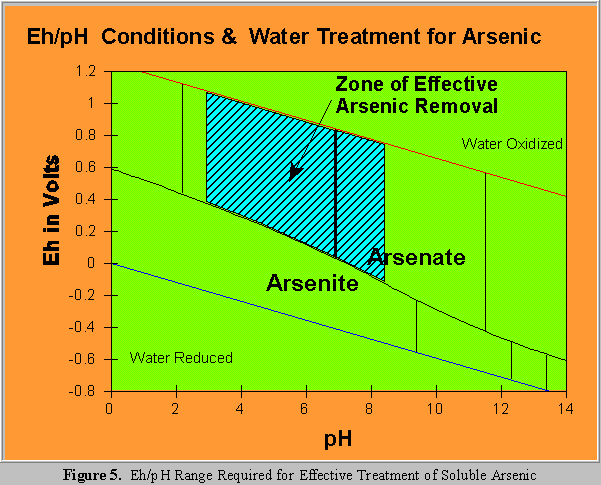
As previously described, the ionization chemistry of arsenic in ground water precludes the removal of arsenite by ion exchange within normal pH ranges. Other technologies including coprecipitation, electrodialysis and reverse osmosis are also affected by arsenite's dissociation profile. One solution to this problem is an oxidation step to form arsenate. Figure 5 illustrates the Eh/pH range required for this process. Oxidation, particularly of drinking water, may be problematic. Chemical residues of the oxidant, by-products from oxidation of other organic or inorganic species, reagent costs, and operational issues are all factors. Oxygen would be ideal, as it is thermodynamically capable of this
oxidizing step. However, the kinetics are exceedingly slow Clifford et al., 1983). Other chemicals can affect arsenite oxidation including free chlorine, hypochlorite, ozone, permanganate, and hydrogen peroxide with ferrous iron.
IRON COPRECIPITATION
Coprecipitation of arsenate with ferric iron is recognized as overall the most effective and practical existing method of arsenic removal. Ferric iron coprecipitation is particularly useful in the mining industry, where large amounts of ferric iron and arsenic can be by-products of production or refining. Adding ferric iron salts for the treatment of drinking water is usually necessary. Figure 4 shows that arsenate is readily removed by iron coprecipitation and the Eh/pH conditions that must be maintained to effect that removal . Due to London Van der Waals bonding, ferric iron coprecipitation of arsenite is also moderately effective with 50% removal at a pH of 7.0 (Pierce and Moore,1982).
The use of iron hydroxides for the coprecipitation of arsenic in industrial wastewater (in which arsenic is in the mg/L range) requires iron dosage 4 to 8 times higher than that of the soluble arsenic, a greater iron dosage yields no further benefit (Krause and Ettel, 1985).
ALUM COPRECIPITATION
Suspended aluminum salts (alum) can remove arsenate via mechanisms similar to those for ferric hydroxides. However, it is less effective over a narrower pH range for arsenate removal and is ineffective for removal of arsenite( Sorg and Logsdon, 1978).
LIME PRECIPITATION
In testing by Nishimura and Tozawa (1985) removal of arsenic with lime precipitation was feasible, but not necessarily practical. High concentrations of arsenic were removed to concentrations of 2 and 4 mg/L for arsenate and arsenite respectively. Removal to lower levels required a second treatment step in which initial arsenic concentrations of 2 mg/L were lowered to 20µg/L for arsenate and 160 µg/L for arsenite. However to achieve these removal efficiencies, the lime dosage was between 5 to 15 grams/L!
ACTIVATED ALUMINA
Activated alumina can be effective for the removal of arsenate under moderately acidic pH conditions (5.5 to 6.0). High levels of competing anions such as sulfate significantly reduce the effectiveness of activated alumina (Frank and Clifford, 1986). The use of activated alumina for complete arsenite removal is ineffective due to the nonionic character of arsenite in that pH range (see Figure 1). Some initial arsenite removal is observed due to London Van der Waals bonding, but compared with arsenate adsorption, this capacity is rapidly exhausted.
ION EXCHANGE
Ion exchange has potential for soluble arsenic removal. Anion exchange resins are available in two basic forms, weak base and strong base. Many weak-base anion exchangers are capable of significant adsorption due to London Van der Waals bonding in addition to ion exchange, giving them a higher level of adsorptive capacity for nonionic arsenite. The author has evaluated anion exchange resins for arsenate removal. The most effective activation was in the hydroxyl form. Chloride and acetate were also tested. Weak base resins had higher loading capacities than strong base (6% vs. 4.8%),), but did not have adequate removal efficiencies (75% vs. 99+% for the strong base resin). Anion exchange resins are also prone to chromatographimg due to the presence of competing anions in the treated water. However, ion exchange is an area of intense research where the development of anionic chelating exchange resins or ion exchange polymers may dramatically improve the technology for arsenic treatment.
REVERSE OSMOSIS
Reverse Osmosis (RO) has been shown to have a removal efficiency greater than 97%. Electrodialysis was only 73% effective. When used to treat 100% arsenite, removal was only 28% (Clifford and Lin, 1991).
OTHER
Rosehart (1972) evaluated a series of removal technologies including activated carbon and sulfide precipitation. Neither performed at a level adequate for use in the treatment of drinking water.
V. CONCLUSIONS
The behavior of arsenic in groundwater and industrial wastewater is dominated by redox and pH conditions. Under a limited range of specific Eh/pH conditions there exists the ability to predict total immobility of arsenic in groundwater and in water treatment systems (see Figures 4 and 5). Except for those conditions arsenic will be partially mobile, the magnitude of which is difficult to predict. Implications of this behavior include:
- Arsenic treatment without control of Eh/pH is likely to be ineffective.
- The injection of water in a reduced oxidation state into sediments with adsorbed arsenic may cause a dramatic mobilization of arsenic into the groundwater.
- If ferric iron sludges used for the coprecipitation of arsenic are disposed under improper Eh/pH conditions arsenic will remobilize.
- In-situ remediation via recovery or stabilization of arsenic contaminated groundwater should be focused on Eh control through chemical or biological methods [1],], [ 2],[3].
Brewster, Michael D.,1992. Removing Arsenic from Contaminated Wastewater, Water Treatment Technology, November, pp 54 to 57.
Calmon, C., 1973. Comment, J. Am. Water Works Assoc., 651, pp. 568-569.
Clifford, D., Ceber, L., and Chow, S., 1983. Arsenic(III)/Arsenic(V) separation by chloride- form ion-exchange resins. XI. Am. Water Works Assoc.Water Qual. Tech. Conf., Norfolk, VA.
Clifford, Dennis and Lin, Chieh-Chien, 1991. Arsenic(III) and Arsenic(V) Removal from Drinking Water in San Ysidro, New Mexico, U.S. EPA Proj. Sum., EPA/600/S2-91/011.
Demayo, A. 1985. Elements in the Earths Crust, In: CRC Handbook of Chemistry and Physics, 66th Edition, Ed. Weast, Robert C., CRC Press Inc., Boca Raton, Fl, p. F145.
Frank, Phyllis and Clifford, Dennis, 1986. Arsenic (III) Oxidation and Removal from Drinking Water, U.S. EPA, EPA-600-52-86/021.
Hem, John D., 1961. Stability Field Diagrams as Aids in Iron Chemistry Studies, Jour. Am. Water Works Assoc., Vol. 53, No. 2, February, 1961,pp. 211-232.
Logsdon, G.S., Sorg, T.J., and Symons, J.M.., 1974. Removal of Heavy Metals by Conventional Treatment, Proceedings of the 16th Water QualityConference, University of Illinois, Urbana, pp. 11-133.
Krause, E. and Ettel, V.A., 1985. Ferric Arsenate Compounds: Are They Environmentally Safe? Solubilities of Basic Ferric Arsenates, In: Proceedings of CIM Metallurgical Society, 15th Annual Hydrometallurgical Meeting, pp5-1 to 5-20.
Nishimura, Tadahisa and Tozawa, Kazuteru, 1985. Removal of Arsenic from Wastewater by Addition of Calcium Hydroxide and Stabilization of Arsenic-Bearing Precipitates by Calcination, In: Proceedings of CIM Metallurgical Society, 15th Annual Hydrometallurgical Meeting, pp 3-1 to 3-18.
Pauling, Linus, 1970. General Chemistry, pp. 499, Dover Publications,Mineola, NY, 959 p.
Pierce, Matthew L. and Moore, Carleton B., 1982. Adsorption of Arsenite and Arsenate on Amorphous Iron Hydroxide, Water Res., Vol. 16, pp. 1247 to 1253.
Robins, R.G., 1985. The Aqueous Chemistry of Arsenic In Relation to Hydrometallurgical Processes, Impurtity Control and Disposal, In: Proceedingsof CIM Metallurgical Society, 15th Annual Hydrometallurgical Meeting, pp1-1 to 1-26.
Rosehart, R. and Lee, J., 1972. Effective Methods of Arsenic Removalfrom Gold Mine Wastes, Can. Min. J., June 1972, pp. 53-57.
Shen, Y.S., 1973. Study of Arsenic Removal from Drinking Water, J. Am. Water Works Assoc. 651, pp. 543-548.
Sorg, T.J., and Logsdon, G.S., 1978. Treatment Technology to Meet the Interim Primary Drinking Water Regulations for Inorganics, Part 2, J. Am.Water works Assoc., Vol. 70, 379-393.
Welch, Alan A., Michael S. Lico, and Hughes, Jennifer L., 1988. Arsenic in Groundwater of the Western United States, Groundwater, Vol. 26, No.3,pp. 333-347, May-June 1988.
Yan Chu, Huang, 1994. Arsenic Distribution in Soils, In: Arsenic in the Environment, Part I: Cycling and Characterization, Ed. Jerome O. Nriagu, John Wiley & Sons, NY, NY, pp. 17-50.
Return to the Environmental Technology Index Page
Return to the 2 The 4 Technology Solutions HomePage
Copyright 2016 David B.Vance
All Rights Reserved
If you have comments or suggestions, email me at dbv7@mindspring.com