|
2 The 4 Technology Solutions

NATURAL ATTENUATION PART III - THE EFFECT OF PUMP AND TREAT REMEDIATION
An On-Line Version of a Column First Published in:
Environmental Technology Nov./Dec. 1996 Vol. 6 No. 6
by: David Vance dbv7@mindspring.com
In-situ groundwater remediation has matured over the past 15 years, particularly with regards to understanding the dynamics of the interactions between contaminants, the impacted saturated soil matrix, and microbiological activity. Recent interest in the phenomena of natural attenuation has served to illustrate the variety of microbial ecosystems that are present in a contaminant plume, each system determined by redox conditions and availability of electron acceptors. While natural attenuation is an attractive alternative to those responsible for groundwater contamination, the regulating communities are more skeptical. The need for proactive groundwater pumping remediation and the efficacy of natural attenuation is a potentially complex balance that is governed by the subsurface conditions of each individual site. There is no universal applicable rule for the resolution of that balance. It is the responsibility of remediation designers to make those site specific determinations and provide the regulating community with information sufficient to support the proactive and the natural attenuation portions of each individual clean-up. The purpose of this column is to point out some of the most significant factors impacting that balance.
The first and most dominant control is the nature of the saturated soil matrix. Four factors are critical:
- The degree and scale of heterogeneity. (1)(2) This determines how much and what portion of an aquifer can be affected with advective groundwater flow. Low permeability regions must rely on diffusional transport, which will dominate the overall remediation rate.
- The time of exposure to the contaminant, this is a direct function of the impact of heterogeneity described above. The contaminant will diffuse into the non-advective portions of the aquifer soil matrix. At minimum remediation will take as long as the initial exposure. Due to adsorptive reactions it is likely to take longer.
- The geochemical composition of the soil matrix. Carbonaceous material and clays have a much higher propensity for the adsorption of organic contaminants. Iron oxides in turn have high adsorptive capacity for metal contaminants. Iron and sulfur minerals may be sources of electron acceptors as redox conditions are modified through the interaction of indigenous microbial populations and the contaminant.
The background geochemical make up of the groundwater as well as that in the contaminant plume. Dissolved oxygen, sulfate, nitrate, and iron can all potentially serve as electron acceptors to aid in the degradation of organic contaminants. Ferrous iron, hydrogen sulfide and carbon dioxide are indicative end products of those reactions.
The distribution of the organic contaminant. Free phase hydrocarbons should be recovered proactively. The treatment of dissolved and adsorbed hydrocarbons is the point at which the balance between proactive remediation and natural attenuation must be determined.
Now, one of the most important contributions that a pump and treat system makes to the in-situ remediation of contaminated groundwater is plume capture in the source zone, and the core of the dissolved and adsorbed plumes. For the purposes of this discussion the most important issue is the background groundwater that is drawn through the plume perpendicular to the natural groundwater flow direction.
In the past the focus of pump and treat remediation has been on how it acts to flush and remove the contaminant. The contributions made by recent developments on the mechanisms of natural attenuation reside in the role of electron acceptors present at background concentrations within the aquifer. From the exterior to the interior of a plume the specific electron acceptor zones are:
- aerobic,
- denitrification, and
- sulfate/iron reducing.
- Methanogenesis also occurs in the core, but that is the subject of another column.
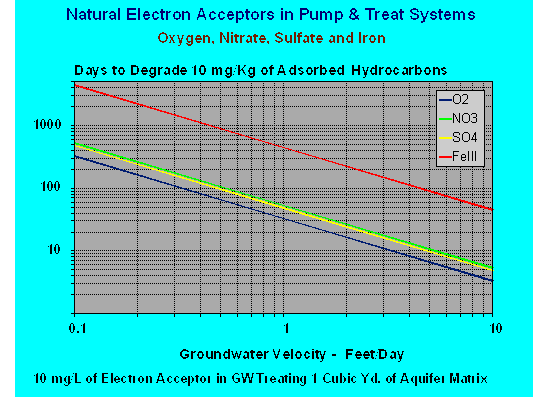
The boundary between each specific redox zone and the active electron acceptor is controlled by the kinetics of the degradation process in each zone and the advective transport rates of groundwater through that zone. In most instances the dominant effect is the groundwater transport rate. To illustrate the point a graph is presented showing groundwater velocity versus hydrocarbon degradation rate stimulated by oxygen, nitrate, sulfate and ferric iron. This graph was prepared with the following assumptions:
- The availability of the respective electron acceptor in the background groundwater is 10 mg/L.
- The mass of hydrocarbon degraded is based on a 10 mg/Kg concentration adsorbed to the aquifer matrix in a volume of one cubic yard.
- The cubic yard of aquifer matrix is treated as a homogenous isotropic block.
- It is assumed that since this data represents groundwater transport induced by a pump and treat system any dissolved phase is removed and recovered with advective groundwater flow.
- Both concentration assumptions were selected for ease of interpolation to other values not presented on the graph.
- The consumption of each electron acceptor is based on the stoichiometry of each respective degradation reaction. The grams of electron acceptor required to degrade one gram of hydrocarbon is:
- 3.1 for oxygen;
- 4.9 for nitrate;
- 4.7 for sulfate; and
- 42 for ferric iron.
- No account is made for the consumption of electron acceptors by extraneous sources.
- Ferrous iron will consume oxygen, and
- in some instances nitrate consumption in the field has exceeded stoichiometric requirements by ten-fold.
In spite of the limitations from the above assumptions, the graph does serve to illustrate the impact of groundwater velocity on the in-situ degradation process. A pump and treat system will increase the groundwater velocities over those occurring naturally. But, permeability constraints and well bores of limited depth will restrict groundwater velocity enhancements in tight soils. However, in permeable soils significant benefit can be gained.
The natural concentrations of these electron acceptors cover a wide range:
- Natural oxygen levels commonly range from 2 to 8 mg/L.
- Groundwater sulfate concentrations in soils derived from sedimentary rocks are typically in the 25 mg/L range, with higher values not uncommon.
- Ferric oxides are commonly present in soils in the range of 0.5 to 5 %.
Given adequate permeability and the presence of appropriate electron acceptors, natural enhancement of pump and treat systems is possible and worth the relatively inexpensive analyses (some of which can be done with field kits) required to evaluate. The next column will look at the transport dynamics of passive natural attenuation systems, which must rely on dispersion and diffusion rather than induced advective flow.
Return to the Environmental Technology Index Page
Copyright 2008 David B. Vance
All Rights Reserved

If you have comments or suggestions, e-mail me at dbv7@mindspring.com
|